Borouge Launches First Made in UAE Healthcare LDPE Solution
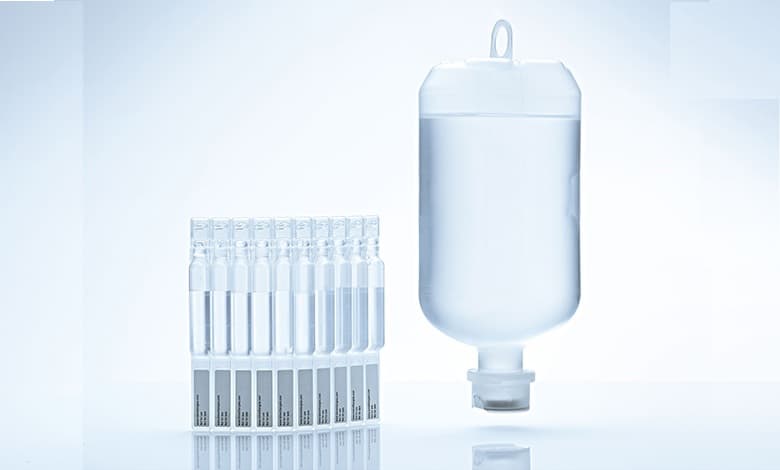
Innovative solution to strengthen medical supply chains across Middle East and Asia; production of low-density polyethylene to enable local manufacturing of IV bottles, ampoules, and other pharma packaging, supporting the ‘Made in the Emirates’ initiative; part of company’s ambition to deliver value-added solutions through local innovation
Abu Dhabi-based polyolefin solutions major Borouge plc has launched the first Made in UAE low-density polyethylene (LDPE) for healthcare applications. The achievement follows the company’s successful debut of its first-ever locally produced healthcare polypropylene (PP) product earlier this year. Manufactured at Borouge’s Ruwais facility, Bormed LE6607-PH marks a significant milestone in strengthening regional medical supply chains and enabling localised production of critical sterile pharmaceutical packaging.
The milestone underscores Borouge’s commitment to advancing industrial self-sufficiency, supporting the ‘Made in the Emirates’ initiative, the national programme to drive industrial growth and economic diversification. It will strengthen the UAE’s healthcare supply chain resilience as Borouge expands its impact in value-added sectors across the Middle East and Asia.
Borouge CEO Hazeem Sultan Al Suwaidi says, “This launch positions Borouge at the forefront of healthcare-grade production in the UAE, strengthening our leadership across the region. By streamlining access to high-quality medical materials, we are proactively advancing our healthcare portfolio with locally manufactured polymers that meet international standards. This strategic move not only supports diverse applications such as pharmaceutical manufacturing and patient care but also reinforces our commitment to innovation and regional growth.”
Leveraging Borealis’ established Bormed technology and long-standing commitment to the healthcare sector, Bormed LE6607-PH is an additive-free LDPE engineered for use in pharmaceutical and medical packaging, including blow-fill-seal (BFS) bottles, ampoules, and other pharmaceutical packaging. The grade is optimised for purity, compliance, and stability, minimising the level of extractables, leachables in final products, and ensuring compatibility with aseptic processing standards. It meets the stringent specifications of both the EU and US Pharmacopoeia.
By manufacturing this grade in the UAE, Borouge supports not just supply chain agility and proximity to key markets, but also consistent quality standards and reliable supply continuity. These advantages help healthcare manufacturers and providers better manage risk, shorten lead times, and ensure patient safety.
The launch builds on the successful introduction of Bormed RG868MO, Borouge’s first UAE-made healthcare product, earlier this year, and now extends the company’s healthcare portfolio even further. Together, these offerings reinforce the petrochemical company’s role as a strategic enabler of medical innovation and national industrial capacity.
With this locally produced polymer, Borouge contributes to safer, more resilient patient care, supports industrial diversification, and strengthens the UAE’s position as a hub for high-quality medical manufacturing.





